
GMDN FOCUS – June 2023
GMDN FOCUS – June 2023 28 June 2023 Click on the link below to download […]
Read More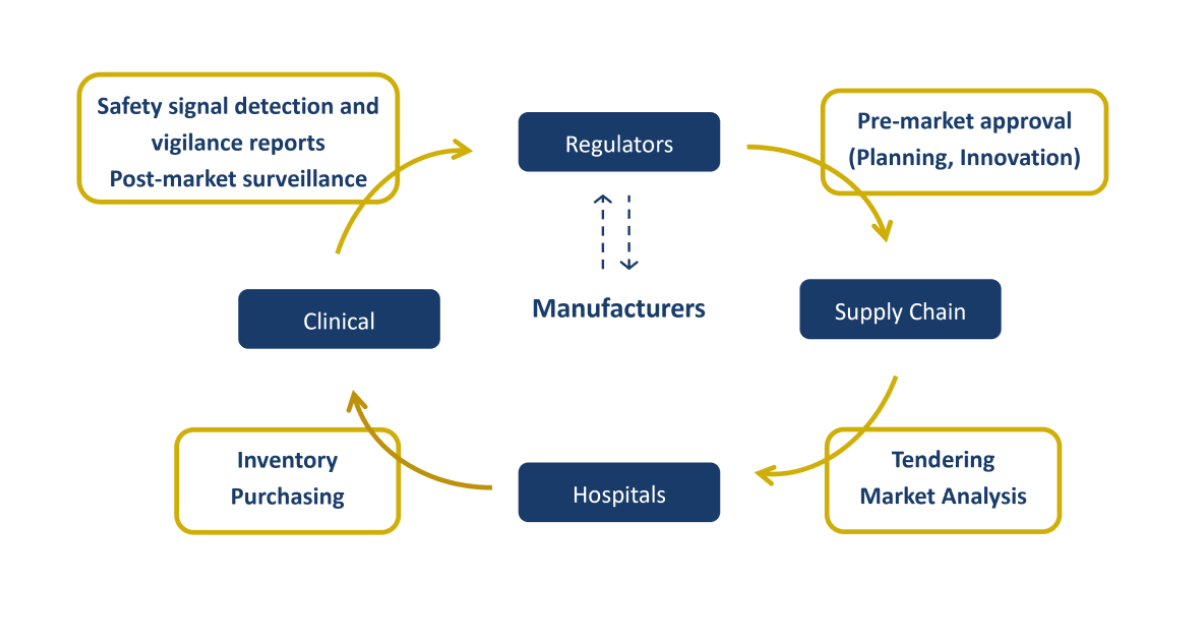
Use Cases of the GMDN
Use Cases of the GMDN 22 June 2023 An article on Use Cases of the […]
Read More
MHRA embeds GMDN in Public Access Registration Database
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) embeds Global Medical Device Nomenclature (GMDN) in Public Access Registration Database
Read More
GMDN Guidance for finding Alternative Terms for Obsolete Terms
GMDN Guidance for finding Alternative Terms for Obsolete Terms 12 June 2023 The GMDN Agency […]
Read More
GMDN Agency attends The MedTech Forum 2023
GMDN Agency attends The MedTech Forum 2023 7 June 2023 Colleagues from The GMDN Agency […]
Read More
Why global harmonisation of medical device nomenclature matters
CEO Blog – Why global harmonisation of medical device nomenclature matters 25 May 2023 CEO […]
Read More
GMDN FOCUS – May 2023
GMDN FOCUS – May 2023 18 May 2023 Click on the link below to download […]
Read More
GMDN Code of Good Practice for Manufacturers
GMDN Code of Good Practice for Manufacturers 16 May 2023 An article about our suggested […]
Read More
GMDN FOCUS – April 2023
GMDN FOCUS – April 2023 15 April 2023 Click on the link below to download […]
Read More