
Guest Blog – The GMDN in a Swedish context
Guest Blog – The GMDN in a Swedish context 8 January 2025 Guest Blog – […]
Read More
Season’s Greetings from the GMDN Agency
GMDN Agency holiday working hours 18 December 2024 Season’s Greetings from the GMDN Agency. Please […]
Read More
GMDN FOCUS – December 2024
GMDN FOCUS – December 2024 18 December 2024 Click on the link below to download […]
Read More
The 2024 GMDN Annual Stakeholder Survey is open
The 2024 GMDN Annual Stakeholder Survey 17 December 2024 The 2024 GMDN Annual Stakeholder Survey […]
Read More
GMDN Agency’s Chinaniso Majoni presents at GHWP 28th Annual Meeting
GMDN Agency’s Chinaniso Majoni presents at GHWP 28th Annual Meeting 12 December 2024 The Global […]
Read More
GMDN FOCUS – November 2024
GMDN FOCUS – November 2024 28 November 2024 Click on the link below to download […]
Read More
Blog – How the Global Medical Device Nomenclature complements UDI Implementation
Blog – How the GMDN complements UDI implementation 26 November 2024 By Edward Glenn, Senior […]
Read More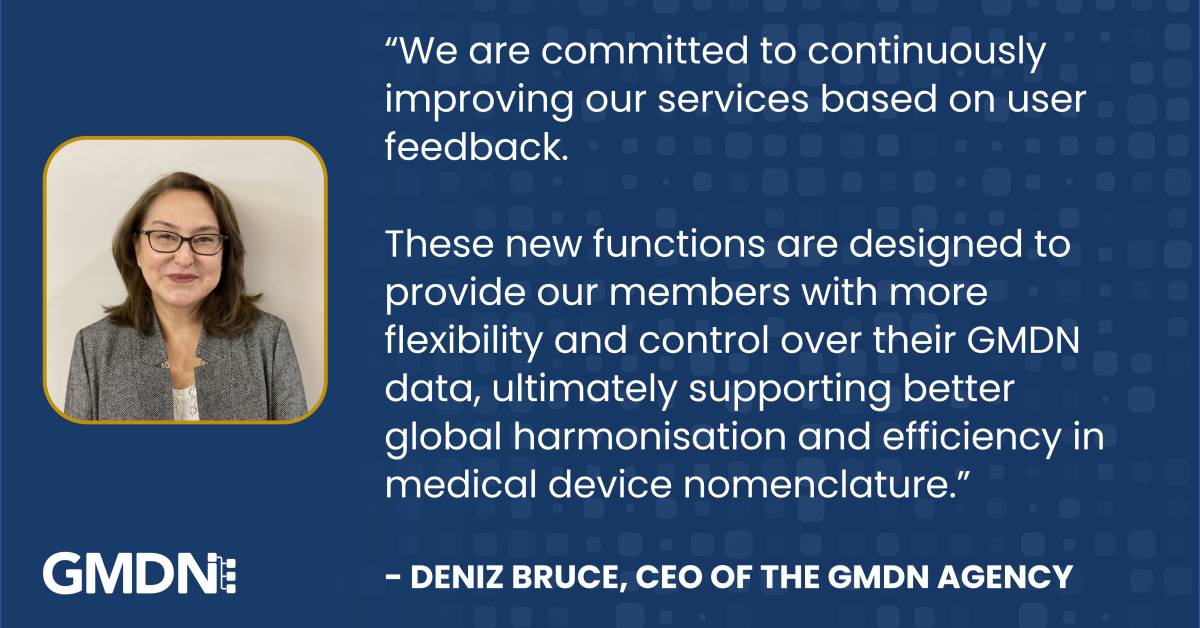
GMDN Agency Launches New Capabilities to Enhance User Experience
GMDN Agency Launches New Capabilities to Enhance User Experience 22 November 2024 The Global Medical […]
Read More
GMDN FOCUS – October 2024
GMDN FOCUS – October 2024 31 October 2024 Click on the link below to download […]
Read More