Blog - Manufacturers: The 'Closest Match' Term Dilemma for Devices

2 July 2025
by Huw Owen, Nomenclature Developer at the GMDN Agency
The Global Medical Device Nomenclature (GMDN) is made up of over 25,000 Terms, covering general medical devices (GMDs) from skin detergents to full body MRI systems and In vitro diagnostics (IVD) as simple as Molecular grade water to those as complex as the analysers on which lab tests are run. With all that diversity it might seem like we should have a Term for just about every medical device you could imagine; but the MedTech sector is a hotbed of development and innovation and the number of devices on the market is growing daily. So, for those devices not yet covered by a GMDN Term what should you, the manufacturer, do? It might seem intuitive to go for the closest match, but in this short piece I will explain the problems that can cause and explain what you can do help us to continue to grow in line with the market (and how it will benefit you in the process)!
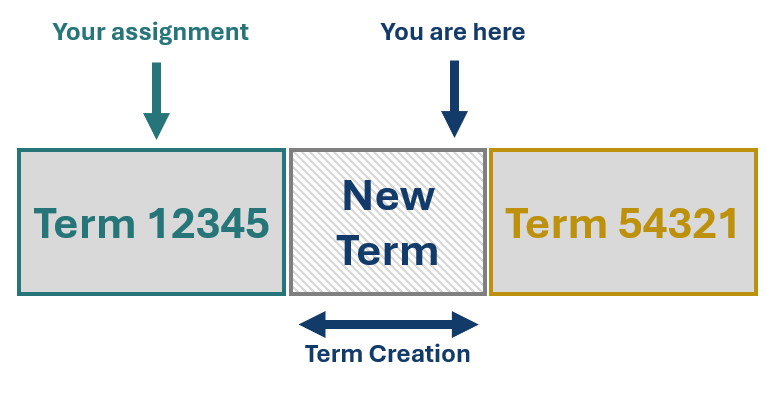
GMDN Terms are written to be mutually exclusive, with no two Terms overlapping, meaning that each device can only be correctly assigned to one Term. The schematic to the right displays two Terms, both of which are close to accurately describing your device, but both have something in the Term name or description which makes them inappropriate. The grey box between these Terms represents an unfilled space in the nomenclature, this is the space where your device fits.
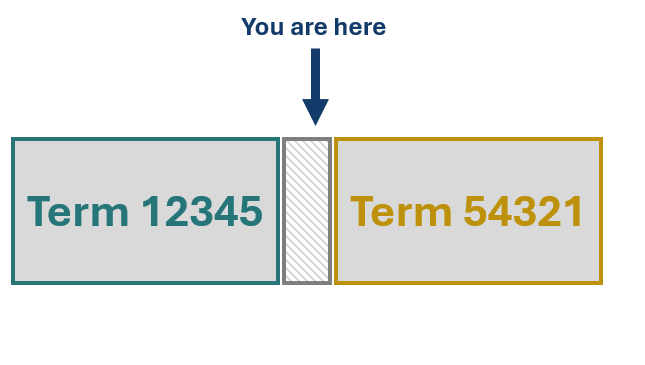
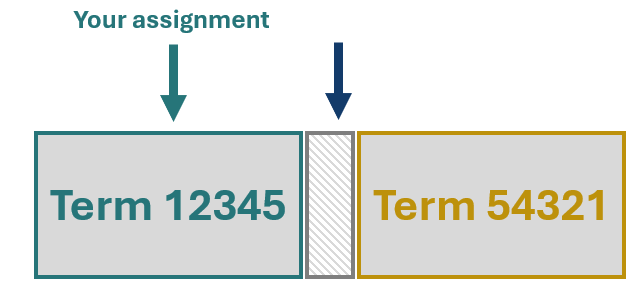
Now, let’s say that you decide to assign to one of these Terms, 12345 in this example, despite the Term name or definition featuring statements which are inaccurate for your device. This means that the gap in the nomenclature remains, and your device is misassigned. We’ll delve deeper into why this is a problem shortly.
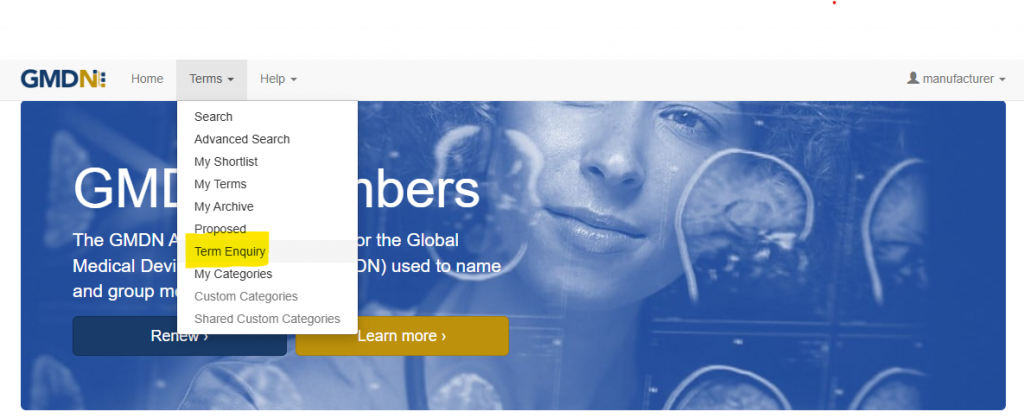
So, what’s the alternative to going for the closest match? Get in touch! Using our ‘Term Enquiry’ tool (https://members.gmdnagency.org/Enquiry) you can contact our Team of nomenclature developers and medical device experts. We will ask you to upload IFU’s, pictures and website links to give us as clear of an understanding of your device as possible.
We will then research your device, search our database to confirm there’s no existing match and from there we will take one of two routes while keeping a clear line of communication to ensure you are happy with our approach:
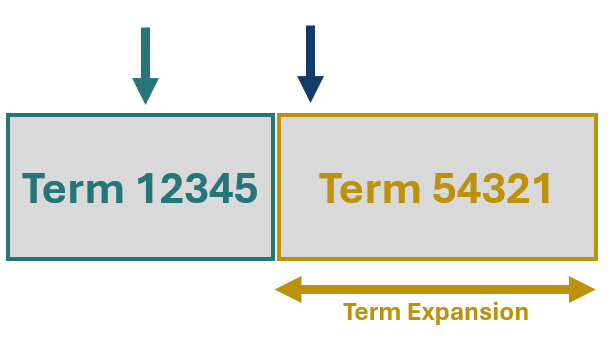
Route one: Term Expansion
Term expansion refers to the process of broadening an existing Term name or definition to accommodate new devices for which the existing Term is accurate except for a few attributes which are unique to the new device. The figure to the right represents Term 54321 being expanded to fill the gap in the nomenclature in which your device (blue arrow) fits. For example, the definition of the GMDN Term ‘General/multiple surgical diode laser system’ was recently expanded from stating the device is ‘…intended to cut, excise, vaporize, and coagulate tissues for general surgery and/or multiple specialized surgical applications (non-dedicated) …’ to ‘…intended to incise, excise, ablate, vaporize and coagulate tissues…’. To many these statements may appear very similar however on closer inspection it becomes apparent that other similar devices were not used for ablation. It would have been easy for this manufacturer to assign their device to the Term as they found it but instead, they came directly to us with their problem, meaning we were able to provide a bespoke expansion, checking in with them along the way to guarantee that their device was correctly assigned.
Route two: Term Creation
Term creation, like Term expansion, is triggered by an enquiry describing a device without an appropriate existing Term; however, in the case of Term creation the new device differs from existing Terms on a more significant attribute (e.g., intended use, use-frequency, clinically relevant materials). The figure to the left represents the creation of a new Term intended to fill the gap in the nomenclature which has been written specifically for your device. Once again, by getting in touch with us, you can be confident your device has a Term to accurately represent it.
But why should correct assignment matter to you? To answer that question let’s go back to our earlier scenario where the device was assigned to a ‘close match’ (green arrow). In that scenario the gap in the nomenclature where the device should fit would remain unfilled until we receive an enquiry which would require us to fill it. One of your competitors, with a device very similar to yours, may get in touch and trigger an expansion of 54321 or the creation of a new Term as described above. Now their device (blue arrow) is correctly assigned to a group with other like devices, while yours is assigned to an inappropriate Term. The GMDN is used for procurement to allow hospitals and procurement groups to compare devices with similar attributes and intended uses. Having your device misassigned means you miss out on the chance to be considered by buyers reviewing that Term. On the other side of the coin, buyers looking at the Term your device is assigned to are unlikely to be interested in your device at that time.
In conclusion, accurate Term assignment is good for us here at the GMDN; it’s good for regulators, and for hospitals, and for patients; but the point of this blog is to let you know it’s also good for you, the manufacturers! We know you’re busy and convenience is important, so if you help us to understand your device, we’ll take it from there and return you an accurate Term, and peace of mind about your assignment accuracy, helping you to find your place in the market.
Recent Posts
- Blog – Manufacturers: The ‘Closest Match’ Term Dilemma for Devices July 2, 2025
- GMDN FOCUS – June 2025 June 30, 2025
- Blog – Reflecting on The Evolution of the Global Medical Device Nomenclature (GMDN) June 27, 2025
- Blog – The Role of GMDN in Reducing Barriers to the Global Medical Device Trade June 4, 2025
- GMDN FOCUS – May 2025 May 30, 2025
